| OCTYL GALLATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 1034-01-1 |
|
| EINECS NO. | 213-853-0 | |
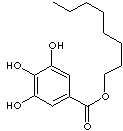
| FORMULA | C6H2(OH)3COOC8H17 | |
| MOL WT. | 282.33 | |
|
H.S. CODE |
2918.29 | |
|
TOXICITY |
|
|
| SYNONYMS | Gallic acid, octyl ester; | |
| Octyl 3,4,5-trihydroxybenzoate; 3,4,5-Trihydroxybenzoic acid, octyl ester; | ||
| SMILES |
|
|
|
CLASSIFICATION |
Antioxidant | |
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white granular |
|
| MELTING POINT | 98 - 101 C | |
| BOILING POINT |
|
|
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
|
|
|
NFPA RATINGS |
|
|
|
AUTOIGNITION |
|
|
|
REFRACTIVE INDEX |
|
|
| FLASH POINT |
|
|
| STABILITY |
Stable under ordinary conditions. |
|
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Gallic acid [3,4,5-trihydroxybenzoic acid, C6H2(OH)3COOH] is obtained from nutgalls and other plants or by the hydrolysis of tannic acid with sulfuric acid. Gallic acid has two functional groups in the same molecule, hydroxyl groups and a carboxylic acid group. They can yield numerous esters and salts including digallic acid which is formed by the reaction of two moles of gallic acid with one another. If gallic acid lose carbon dioxide, pyrogallol is formed. Gallic acid and its derivatives are used in making dyes and inks, photographic developers and has been used as astringents in medically. Some gallates are used as antioxidants in foods. Tannins (tannic acid or gallotannic acid) are esters of digallic acid. They are widely used in tanning leather, dyeing fabrics, manufacturing inks, and in various medical applications (as astringents and to treat some skin diseases). Benzoic acid derivatives substituted by hydroxyl group or ether containing oxygen atom have bacteriostatic and fragrant properties. They are typically used in pharmaceutical and perfumery industry. The destructive metabolic property of oxygen containing benzoic acid derivatives such as protocatechuic acid (3,4-dihydroxybenzoic acid) and veratric acid (3,4-dimethoxybenzoic acid) is used in the application for pharmaceuticals. Protocatechuic acid is a catabolite of epinephrine. Prazosin, a peripheral vasodilator and antihypertensive, is also an example of the application of veratric acid. Hydroxy and ether substituted benzoic acids feature analogue metabolite of aspirin (acetylsalicylic Acid). They are used as intermediates for pharmaceuticals (especially for antipyetic anlgesic, antirheumatism) and other organic synthesis. They are used as matrix for ionization of peptides, proteins and carbohydrates. | ||
| SALES SPECIFICATION (BP, Ph Eur) | ||
|
APPEARANCE |
white granular |
|
|
ASSAY
|
97.0 - 102.0% |
|
|
IDENTITY |
pass |
|
|
FREE
ACID
|
pass |
|
|
Cl |
100ppm max |
|
|
ASH |
0.1% max |
|
|
HEAVY METALS |
10ppm max |
|
| TRANSPORTATION | ||
| PACKING |
25kgs in fiber drum |
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XN, Risk Phrases: 22-43, Safety Phrases: 24-37 | ||